In the Unites States, the Chicago Department of Public Health released a health alert on May 9, 2023, with the following details:
- From April 17 to May 5, 2023, there were 12 confirmed and 1 probable case of Mpox reported, all among symptomatic men, at least 2 PLHIV
- 9 of 13 cases (69%) were among men who were fully vaccinated for Mpox; cases with previous vaccine had mild disease, 1 proctitis
- CDC has stated they have not identified clusters in other communities outside of Chicago at this time
The Emergency Committee acknowledged the progress made in the global response to the multi-country outbreak of mpox and the further decline in the number of reported cases since the last meeting. The Committee observed that a few countries continued to see a sustained incidence of illness; the Committee is also of the view that underreported detection and under-reporting of confirmed cases of illness in other regions is likely. Therefore, the Committee considered various options to sustain attention and resources to control the outbreak and advised maintaining the Public Health Emergency of International Concern (PHEIC), while beginning to consider plans to integrate mpox prevention, preparedness and response within national surveillance and control programmes, including for HIV and other sexually transmissible infections.The WHO European Region reported that as of 3 February, 43 countries and territories have not detected any new cases in the past three months. While 18 countries and territories continue to report recent local human-to-human transmission, case numbers have decreased significantly. Future risks of outbreaks relate to the ongoing importation, forthcoming mass gatherings, potential reduced vaccination and surveillance, limited access to testing and behaviour change/. To tackle this, the Region is working towards a five-year plan to achieve and sustain the elimination of mpox in all Member States through engagement with affected communities and integrating intervention into the sexual health programs, to be discussed at the Regional Committee in autumn 2023.The Region of the Americas reported a stable number of cases in the last six weeks, with 200-250 cases per week, and 4% of cases occurring in women. In addition, while the vaccine supply is limited, seven countries have started vaccination. Risk communication and community engagement interventions are being delivered through HIV community-based networks.The Committee reconvened in a closed meeting to examine the questions in relation to whether the event continues to constitute a PHEIC, and if so, to consider the proposed Temporary Recommendations, drafted by the WHO Secretariat in accordance with IHR provisions. The Secretariat provided a presentation on the legal provisions under the IHR in relation to the determination of a PHEIC, and the issuance of Temporary Recommendations.
Updated January 31
HIV.gov convened a meeting of federal HIV communications leadership to start the new year off with the critical message that integrating mpox messaging into our ongoing communications is foundational to our HIV response for 2023. While we recognize the important work that has been done to dramatically decrease new mpox cases, we cannot take our foot off the pedal, as there is still critical work needed to increase and routinize mpox vaccinations.
We were fortunate to hear about mpox in the context of HIV and the importance of an equity-centered response from White House and other U.S. Government leaders. Demetre C. Daskalakis, MD, MPH, Director, CDC Division of HIV/AIDS Prevention and Deputy Coordinator, White House National Mpox Response noted that mpox continues to be a public health issue that disproportionately impacts people with HIV, and data suggest that approximately 40% of people diagnosed with mpox in the United States also have HIV. He also highlighted important action steps (see below) and resource videos to move us forward.

Beginning the meeting, Kaye Hayes, MPA, Deputy Assistant Secretary for Infectious Disease and Director of the Office of Infectious Disease and HIV/AIDS Policy, emphasized the Biden-Harris Administration’s focus on the importance of equity in the mpox response to ensure that no communities are left behind. She also highlighted the intentionality of the work surrounding mpox, including hearing directly from populations most likely to be affected by mpox but least likely to be vaccinated to better understand what is working and what needs to be improved in our response.
Decline in Mpox Cases
During the meeting, Dr. Daskalakis noted that in the U.S., there has been around a 99% reduction in the number of daily mpox cases since the peak of the mpox outbreak in summer 2022. He attributed this to 1) effective communications to gay, bisexual, and other men who have sex with men, as well as transgender individuals and other gender-diverse individuals; 2) the mpox vaccine; and 3) swift response from the LGBTQI+ community.
Syndemics and Mpox
Dr. Daskalakis also discussed mpox in the context of syndemics, noting that mpox infection does not occur in isolation. The September 2022 CDC Morbidity and Mortality Weekly Report showed that HIV or recent sexually transmitted infections (STIs) are common among people with mpox. Dr. Daskalakis thus stressed the importance of continuing to ensure equitable access to mpox screening, prevention, and treatment, including both prioritizing people with HIV and STIs for mpox vaccination and offering HIV and STI screening for people evaluated for mpox. He also emphasized the need to encourage those who haven’t received their second vaccinations to do so.
“We cannot take mpox in isolation,” he asserted. “We need to put it in the context of the interacting epidemics and the interacting social determinants of health that make mpox worse, or that propel mpox transmission.”
The Way-Forward
Dr. Daskalakis discussed navigating the future of the domestic response to mpox. As of the time of the meeting, there were 1,152,073 U.S. mpox vaccines administered. To get to zero mpox cases, he noted we must focus on communications to increase mpox vaccinations and magnify our communications for mpox vaccine administration. [Note: imagery promoting this blog will include this statement]. He also encouraged increased engagement with partners via social media, as well as official government websites, such as HIV.gov.
Harold Phillips, Director, The White House Office of National AIDS Policy, offered a closing message to attendees. He noted that the mpox response was “a true demonstration of when we use and follow the data and the science and we center the approach with equity, we CAN make a difference.”
Stay up to date on mpox and view the CDC’s Mpox Vaccine Equity Toolkit and their Cases and Data page. Also watch and share HIV.gov’s mpox videos featuring Dr. Daskalakis answering 14 top mpox questions.
Updated January 23
Today, the CDC team updated resources related to mpox. These may be found below
New and Updated CDC Resources:
MMWR: Epidemiology of Human Mpox — Worldwide, 2018–2021 NEW
Strategies for Talking with Patients about Vaccinations for Mpox UPDATED
Autopsy and Handling of Human Remains of Patients with Mpox UPDATED
What’s New & Updated UPDATED
Additional Funding Resources:
Mpox Guidance for CDC Grant Recipients
Mpox Considerations for Sexual Health Services (Dear Colleague Letters)
HRSA: Use of Ryan White HIV/AIDS Program Funds for Mpox
HUD’s HOPWA (Housing Opportunities for Persons with AIDS)
SAMHSA: Dear Colleague Letter on Using SAMHSA Grant Resources for Mpox-related Activities
Recently Updated—In Case You Missed It:
MMWR: Mpox Cases Among Cisgender Women and Pregnant Persons — United States, May 11–November 7, 2022
Data and Analytics:
2022 U.S. Mpox Outbreak UPDATED
U.S. Map & Case Count UPDATED
U.S. Mpox Case Trends Reported to CDC UPDATED
Global Map & Case Count UPDATED
Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms UPDATED
Mpox Vaccine Administration U.S. Map UPDATED
Demographics of Patients Receiving TPOXX for Treatment of Mpox UPDATED
Mpox Technical Reports
Additional CDC Resources:
CDC’s Mpox Internet Site
MMWR Mpox Reports
Health Alert Network (HAN)—Severe Manifestations of Mpox
Mpox Vaccine Confidence Insights Report
Mpox Vaccine Equity Pilot Program
Science Brief: Detection and Transmission of Mpox Virus
Clinician FAQs
Mpox Vaccination Program Provider Agreement
Clinician Outreach and Communication Activity (COCA) Call
Clinical Considerations for Treatment and Prophylaxis of Mpox Virus Infection in People with HIV
Additional Intradermal Administration Sites: JYNNEOS Vaccine
Video: How to Administer Intradermal Vaccine in Forearm, Deltoid, and Scapula
Videos on mpox recommendations and updates from CDC leadership and partners
Completing a Death Certificate in the Setting of Mpox
V-safe after Vaccination Health Checker for Mpox Vaccine
V-safe Print Materials
Mpox Toolkit for Correctional and Detention Facilities
Safer Sex, Social Gatherings, and Mpox
Strategies for Talking with Patients about Vaccinations for Mpox
CDC’s Vaccine Equity Efforts in the Peach State- The Atlanta Black Pride Story
Stories from the Mpox Response
CDC-INFO On Demand – Publications
Print Resources
Additional Partner Resources:
Mpox Vaccine Locator (mpoxvaxmap.org)
CDC/IDSA Clinician Call: Updates & Emerging Issues on COVID-19 and Mpox
ASPR: JYNNEOS Mpox Vaccine Distribution by Jurisdiction
ASPR: Operational Planning Guide
Clinicaltrials.gov: STOMP
Study of Tecovirimat for Human Mpox Virus (STOMP)
FDA: Emergency Use Authorization Fact Sheet
HHS amends PREP Act declaration increasing workforce authorized to administer mpox vaccines
HHS: Public Readiness and Emergency Preparedness (PREP) Act Coverage for Mpox
HHS: Statement From HHS Secretary Becerra on mpox
HHS: U.S. Government Mpox Research Summary
HIV.gov: Addressing Mpox Holistically
HIV.gov: Mpox and People with HIV Videos
NIH: U.S. Clinical Trial Evaluating Antiviral for Mpox Begins
PREP Act Coverage Frequently Asked Questions for Mpox
SAMHSA: Anxiety and Stress Related to Mpox
The White House: A Comprehensive Summary of Federally-Funded Mpox Research Projects
The White House: Mpox Press Briefing (9/28/2022)
WHO: Clinical Management and Infection Prevention and Control of Mpox
WHO: Community Engagement
WHO: WHO recommends new name for monkeypox disease
Published in MMRW today
HIV and Sexually Transmitted Infections Among Persons with Monkeypox — Eight U.S. Jurisdictions, May 17–July 22, 2022
Summary
What is already known about this topic?
In the current global monkeypox outbreak, HIV infection and sexually transmitted infections (STIs) are highly prevalent among persons with monkeypox.
What is added by this report?
Among 1,969 persons with monkeypox in eight U.S. jurisdictions, 38% had HIV infection, and 41% had an STI in the preceding year. Among persons with monkeypox, hospitalization was more common among persons with HIV infection than persons without HIV infection.
What are the implications for public health practice?
It is important to leverage systems for delivering HIV and STI care and prevention and prioritize persons with HIV infection and STIs for vaccination. Screening for HIV and other STIs and other preventive care should be considered for persons evaluated for monkeypox, with HIV care and HIV preexposure prophylaxis offered to eligible persons.
Monkeypox and HIV
CDC doesn’t know if having HIV increases the likelihood of getting monkeypox. Monkeypox can spread to anyone through prolonged, close, personal, often skin-to-skin contact, as well as through contact with objects, fabrics (clothing, bedding, or towels), and surfaces that have been used by someone with monkeypox, or contact with respiratory secretions, through kissing and other face-to-face contact.
CDC continues to monitor monkeypox among people with HIV. During the current monkeypox outbreak, there does not appear to be more severe monkeypox illness in people who have HIV and are virally suppressed (having less than 200 copies of HIV per milliliter of blood). In fact, the World Health Organization (WHO) monkeypox guidance states, “People living with HIV on antiretroviral therapy with suppressed viral load are not considered to be immunosuppressed.” However, people with HIV who are not virally suppressed may be at increased risk for severe illness and death from monkeypox.
Currently there is no treatment approved specifically for monkeypox. However, medicine (antivirals) developed for use in patients with smallpox may help treat people with monkeypox.
At this time, CDC doesn’t have enough data to know whether people who have HIV and are virally suppressed might benefit from taking medicine if they get monkeypox.
Because patients with a weakened immune system may have more severe monkeypox illness, healthcare providers might consider using antiviral medicines (e.g., tecovirimat) or Vaccinia Immune Globulin for these patients. This could include people newly diagnosed with HIV or people with HIV who are not virally suppressed. See: Treatment Information for Healthcare Professionals.
At this time, vaccination is recommended for people with exposures to a probable or confirmed case with monkeypox, for example people who have had close physical contact with someone diagnosed with monkeypox. Vaccination may also be offered to people who had a presumed exposure, such as men who have sex with men who have had multiple sexual partners during the past 14 days in a jurisdiction with known monkeypox activity.
There are currently two licensed vaccines in the United States to prevent smallpox – JYNNEOS and ACAM2000. These smallpox vaccines may provide protection against monkeypox because smallpox and monkeypox are very similar viruses. Only JYNNEOS is FDA approved for the prevention of monkeypox in people 18 and older.
The JYNNEOS vaccine has been studied in people with HIV who are virally suppressed, and they do not have more frequent or severe side effects from the vaccine than people who did not have HIV. The JYNNEOS vaccine seems to be well tolerated, with the most common side effects being injection site pain, redness, swelling and itching. Some recipients also reported muscle pain, headache, fatigue, nausea, and chills. More data are needed to know if this vaccine is tolerated by people newly diagnosed with HIV or by people with HIV who are not virally suppressed. Clinicians should weigh the benefits of vaccination with the unknown risk of an adverse event for a person if their HIV is not virally suppressed.
ACAM2000 has been shown to have more frequent and severe side effects, especially for people with weakened immune systems or who are pregnant, have a heart condition, or skin conditions like eczema, psoriasis, or dermatitis. ACAM2000 is not recommended for people with HIV, even if they are virally suppressed, due to this increased risk of severe side effects.
Data is limited, but most HIV treatment can be safely given with monkeypox treatment and smallpox vaccines. People with HIV should inform their healthcare provider of all their medications to help determine if any interactions exist.
People with HIV should follow the same recommendations as everyone else to protect themselves from monkeypox.
- Avoid direct contact with rashes, sores, or scabs on a person with monkeypox, including during intimate contact such as sex. We believe this is currently the most common way that monkeypox is spreading in the U.S.
- Avoid contact with objects, fabrics (clothing, bedding, or towels), and surfaces that have been used by someone with monkeypox.
- Avoid contact with respiratory secretions, through kissing and other face-to-face contact from a person with monkeypox.
New Study Documents the Frequent Detection of Monkeypox Virus DNA in Saliva, Semen, and other Clinical Samples
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2022.27.28.2200503
This week, the administration released an new fact sheet on Monkey pox. Key is the rollout of a national strategy for smallpox vaccination against monkeypox for people at risk. This follows a decision by Quebec Province to do the same with that province and Montreal being the epicenters of the current outbreak in North America
https://www.whitehouse.gov/briefing-room/statements-releases/2022/06/28/fact-sheet-biden-harris-administrations-monkeypox-outbreak-response/
As part of the monkeypox outbreak response, the Biden-Harris Administration is launching a national strategy to provide vaccines for monkeypox for individuals at higher risk of exposure. The strategy aims to mitigate the spread of the virus in communities where transmission has been the highest and with populations most at risk. This plan distributes the two-dose JYNNEOS vaccine, which the Food and Drug Administration (FDA) approved for protection against smallpox and monkeypox in individuals 18 years and older determined to be at high risk for smallpox or monkeypox infection. States will be offered an equitable allotment based on cases and proportion of the population at risk for severe disease from monkeypox, and the federal government will partner with state, local, and territorial governments in deploying the vaccines.
The goal of the initial phase of the strategy is to slow the spread of the disease. HHS will immediately allocate 56,000 vaccine doses currently in the Strategic National Stockpile to states and territories across the country, prioritizing jurisdictions with the highest number of cases and population at risk. To date, vaccines have been provided only to those who have a confirmed monkeypox exposure. With these doses, CDC is recommending that vaccines be provided to individuals with confirmed monkeypox exposures and presumed exposures. This includes those who had close physical contact with someone diagnosed with monkeypox, those who know their sexual partner was diagnosed with monkeypox, and men who have sex with men who have recently had multiple sex partners in a venue where there was known to be monkeypox or in an area where monkeypox is spreading.
In the coming weeks, HHS expects to receive an additional 240,000 vaccines, which will be made available to a broader population of individuals at risk. HHS will hold another 60,000 vaccines in reserve.
HHS expects more than 750,000 doses to be made available over the summer. An additional 500,000 doses will undergo completion, inspection, and release throughout the fall, totaling 1.6 million doses available this year.
First Case Report of Monkeypox in a Person Living with HIV
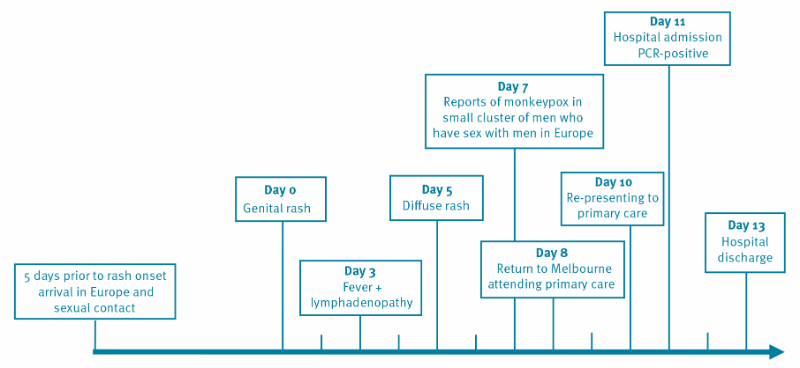
An HIV-positive man in his 30s taking Abacavir, Lamivudine and Dolutegravir and with a CD4 + T-cell count above 700 cells/mm3 (normal range 410–1,545 cells/mm3) and HIV viral load < 100 copies/mL, visited a primary care doctor after his return from Europe to Melbourne, Australia. He reported onset of a genital rash 8 days earlier. The rash had started 5 days after he reported unprotected sex with four casual male partners in Europe. The initial symptoms were painless white pustules on the penis that became painful and pruritic. He reported that he developed a fever and malaise 3 days after the first appearance of the penile rash and over the subsequent 5 days, the rash disseminated to his trunk, then more sparingly to the face and limbs while the genital lesions crusted over.
Swabs taken from deroofed skin lesions on the hand, calf and trunk in addition to combined nose throat swabs on the day of hospital admission, were all positive for monkeypox virus using previously described conventional [2] and in-house RT-PCR assays for orthopox and monkeypox viruses. Whole genome sequencing performed as described in the Supplementary material of DNA derived from the skin lesions resulted in the complete recovery of the entire monkeypox genome (MPXV-VIDRL01, Genbank_ID ON631963) with phylogenetic analysis revealing clustering with other monkeypox virus sequences from the May 2022 outbreak in Europe and the United States.
Rapid communication Home Euro surveillance Volume 27, Issue 22, 02/Jun/2022 Monkeypox infection presenting as genital rash, Australia Yael Hammerschlag et al
|
The World Health Organization (WHO) reported in its May 30, 2022, update that it had received reports of 257 confirmed monkeypox cases and approximately 120 suspected cases in 23 countries where the virus is not endemic as of May 26, 2022. In the United States, the Centers for Disease Control and Prevention (CDC) has reported 12 cases in eight states as of May 27, 2022. No deaths have been reported in nonendemic countries. The WHO classifies the global public health risk level posed by monkeypox as moderate.
|
Background
Monkeypox was first detected in 1958 in laboratory monkeys.1 The first human case of monkeypox was recorded in 1970 in the Democratic Republic of Congo.2 Since then, monkeypox has been reported in humans in other central and western African countries, with occasional cases reported outside of Africa.1
Global Outbreak
In May 2022, more than 120 confirmed or suspected cases of monkeypox have been reported in at least 11 non-African (endemic) countries, including Australia, Belgium, Canada, England, France, Germany, Israel, Italy, Netherlands, Portugal, Spain, Sweden, and Switzerland.2
Historical Context
In 2003, the first monkeypox outbreak outside of Africa was in the United States, when 70 cases in humans were reported, linked to contact with infected pet prairie dogs, which had been housed with Gambian pouched rats imported into the United States from Ghana.3 Monkeypox was reported in travelers from Nigeria to the United States in July 2021 and November 2021.3
2022 US and Global Outbreak
On May 20, 2022, the US Centers for Disease Control and Prevention (CDC) issued an alert urging doctors and state health departments to be vigilant for cases of monkeypox, following confirmation of cases in the US.4, 5 Federal officials say they expect to identify additional infections in the coming days. According to the CDC, it is not clear how people in the cluster outbreaks so far were exposed to the monkeypox virus but cases include people who self-identify as men who have sex with men.6 Public health officials have issued similar alerts in Australia, Belgium, Canada, England, France, Germany, Israel, Italy, Netherlands, Portugal, Spain, Sweden, and Switzerland.
Monkeypox virus is known to spread through close contact with the lesions, bodily fluids and respiratory droplets of infected people or animals or materials contaminated with the virus. Human transmission is thought to occur primarily through respiratory droplets. Investigations are ongoing that the virus may be spreading by sexual contact, following outbreaks of monkeypox in Europe related to two parties in Spain and Belgium, attended primarily by gay men. Although many cases have been reported among men who have sex with men (MSM), and bisexual men, spread may be occurring because the virus was introduced into the community and it has continued to spread there, both by sexual and social contact.
Key Facts about Monkeypox3
- Monkeypox is caused by monkeypox virus.
- Monkeypox typically presents clinically with fever, rash, and swollen lymph nodes and may lead to a range of medical complications.7
- The incubation period is usually 7-14 days but can range from 5-21 days.
- Monkeypox is usually a self-limited disease with the symptoms lasting from 2 to 4 weeks. Severe cases can occur and the fatality ratio has been around 3-6%.
- Monkeypox is transmitted to humans through close contact with an infected person (skin lesions, body fluids, respiratory droplets and contaminated materials such as bedding) or animal, or with material contaminated with the virus.
- The clinical presentation of monkeypox resembles that of smallpox but is less contagious than smallpox and causes less severe illness.
- Vaccination against smallpox was demonstrated through several observational studies to be about 85% effective in preventing monkeypox. A vaccine based on a modified attenuated vaccinia virus (Ankara strain) was approved for the prevention of monkeypox in 2019.
- An antiviral agent (tecovirimat) that was developed for smallpox was licensed by the European Medical Association (EMA) for monkeypox in 2022 based on data in animal and human studies. Tecovirimat is not yet widely available.
Summary of CDC Recommendations for Clinicians6
- If clinicians identify patients with a rash that could be consistent with monkeypox, especially those with a recent travel history to areas reporting monkeypox cases, monkeypox should be considered as a possible diagnosis.
- The rash associated with monkeypox involves vesicles or pustules that are deep-seated, firm or hard, and well-circumscribed
- Presenting symptoms typically include fever, chills, the distinctive rash, or new lymphadenopathy; however, onset of perianal or genital lesions in the absence of subjective fever has been reported.
- Information on infection prevention and control in healthcare settings is provided on the CDC website’s Infection Control page.8
- Clinicians in the United States should consult their state health department or CDC through the CDC Emergency Operations Center (770) 488-7100 as soon as monkeypox is suspected.
- Clinicians outside of the United States consult their relevant subnational and national public health authorities for guidance and epidemiological surveillance purposes.
What At-Risk Individuals Should Do6
The CDC advises people who may have symptoms of monkeypox should contact their healthcare provider. This includes anyone who:
- Traveled to central or west African countries, parts of Europe where monkeypox cases have been reported
- Reports contact with a person with confirmed or suspected monkeypox
The World Health Organization (WHO) notes that available evidence suggests that those who are most at risk are those who have had close physical contact with someone with monkeypox, and that risk is not limited to men who have sex with men.
Notes
[1] Monkeypox goes global: why scientists are on alert Max Kozlov Nature News May 20 2022
[2] CDC Monkeypox https://www.cdc.gov/poxvirus/monkeypox/index.html Last updated May 20 2022
[3] Monkeypox World Health Organization May 19,2022 https://www.who.int/news-room/fact-sheets/detail/monkeypox
[4] CDC tells doctors to be on alert for monkeypox as global cases rise Washington Post https://www.washingtonpost.com/health/2022/05/20/cdc-monkeypox-alert/
[5] Monkeypox in the United States CDC https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html
[6] 2022 United States Monkeypox Case https://www.cdc.gov/poxvirus/monkeypox/outbreak/current.html
[7] Signs and Symptoms CDC https://www.cdc.gov/poxvirus/monkeypox/symptoms.html
[8] Precautions to Prevent Monkeypox Transmission https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-hospital.html







 Over the past four weeks, Ukraine has become the focal point of international attention as it pushes back on Russian military aggression while enduring the hardships that war inflicts on combatants and civilians. In the images depicting the effects of military assaults, it is difficult to differentiate the people who are feeling the impact of an unprovoked war. Nonetheless, among them are Ukrainians living with HIV and people who are vulnerable to HIV acquisition based on social determinants of health. Dr. José M. Zuniga, President/CEO of the International Association of Providers of AIDS Care (IAPAC) and the Fast-Track Cities Institute (FTCI), reflects upon the humanitarian crisis and efforts to show solidarity with the people of Ukraine.
Over the past four weeks, Ukraine has become the focal point of international attention as it pushes back on Russian military aggression while enduring the hardships that war inflicts on combatants and civilians. In the images depicting the effects of military assaults, it is difficult to differentiate the people who are feeling the impact of an unprovoked war. Nonetheless, among them are Ukrainians living with HIV and people who are vulnerable to HIV acquisition based on social determinants of health. Dr. José M. Zuniga, President/CEO of the International Association of Providers of AIDS Care (IAPAC) and the Fast-Track Cities Institute (FTCI), reflects upon the humanitarian crisis and efforts to show solidarity with the people of Ukraine.








 “IAPAC welcomes the Irish cities of Cork, Dublin, Galway, and Limerick to the Fast-Track Cities network, and we applaud the political commitment made by their Mayors, in partnership with affected communities, to work towards ending their local HIV epidemics,” said Dr. Zuniga. “We also applaud the Department of Health’s investment towards Fast-Track Ireland activities – an investment that is ‘wind in the sails’ for ambitious plans to scale-up HIV testing, linkage to preventative and therapeutic care, and eliminate the barrier that AIDS-related stigma continues to represent almost four decades into the global HIV epidemic.”
“IAPAC welcomes the Irish cities of Cork, Dublin, Galway, and Limerick to the Fast-Track Cities network, and we applaud the political commitment made by their Mayors, in partnership with affected communities, to work towards ending their local HIV epidemics,” said Dr. Zuniga. “We also applaud the Department of Health’s investment towards Fast-Track Ireland activities – an investment that is ‘wind in the sails’ for ambitious plans to scale-up HIV testing, linkage to preventative and therapeutic care, and eliminate the barrier that AIDS-related stigma continues to represent almost four decades into the global HIV epidemic.”




